Take good care of your liver as it can influence brain function

BY ASSOC PROF DR ROSLINA ABDUL RAHIM
Non-alcoholic fatty liver disease (NAFLD) is a pathological condition where fat accumulation in the liver hepatocytes. It is also known as hepatic steatosis.
NAFLD is a common liver disease resembling alcoholic liver disease (ALD), clinically and histologically, but it occurs in people who consume little or no alcohol.
Meanwhile, non-alcoholic steatohepatitis (NASH) is the progressive inflammatory form of NAFLD, with histological features of hepatocyte injury (ballooning) and inflammation, with or without fibrosis. The gold standard for diagnosing NASH is a liver biopsy and histological scoring.
The prevalence of NAFLD and NASH are increasing not only in western countries but also Malaysia affecting all ethnics and younger and older adults according to Malaysian Society of Gastroenterology and Hepatology. This disease has been recognized as main health problem.
Patients with NASH are usually asymptomatic, but it can gradually progress to cirrhosis, end-stage liver disease and has an increased risk of developing hepatocellular cancer (HCC). NASH is strongly associated with hypertriglyceridaemia, truncal obesity (fat around the stomach and abdomen), Type 2 diabetes and metabolic syndrome.
This is mainly due to lipotoxicity-associated disturbance in insulin activity and signalling, mitochondrial dysfunction, and oxidative stress, with dysregulation of lipid transport and metabolism in adipose tissue.
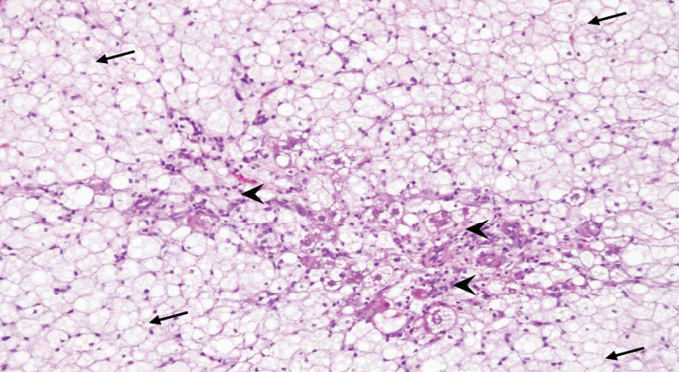
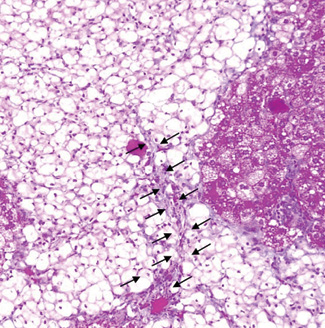
Many animal models are used to study NASH. The figure 1 and 2 as below are showing high cholesterol diet induced NASH in animal model currently being done to further study molecular mechanism of NASH (Alias et al, 2022; IMJM).
Cognitive dysfunction
According to the National Institute of Diabetes and Digestive and Kidney Diseases, NAFLD and NASH are silent diseases with few or no symptoms. The symptoms are discomfort on the right side of the abdomen and fatigue or tiredness.
As the disease progresses in cirrhosis, the patient will suffer from ascites (abdominal swelling), enlarged blood vessels beneath the skin surface, enlarged spleen, red palms, yellowish skin, and eyes (jaundice). Some studies reported that NASH patients also suffer cognitive dysfunction and depression, which affect the prefrontal cortex of the brain and hippocampus degeneration.
Cognitive dysfunction is described as a loss of intellectual thinking, remembering, and reasoning functions that interfere with daily functioning. Patients with cognitive dysfunction also have trouble with verbal recall, basic arithmetic, and concentration. Depression is a common and severe medical condition affecting how you think, act and feel negative.
Multivariate data analysis also showed that patients with severe hepatocytes ballooning suffer mood disorders. An increase in serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), gamma-glutaryl transpeptidase (GGT) and high sensitive C-reactive protein (hs-CRP) can also show major depressive disorders.
The central nervous system (CNS) is protected by the blood-brain barrier (BBB), which consists of non-fenestrated endothelial cells with a tight junction between them. Meanwhile, cytokines are large, hydrophilic molecules that cannot readily cross the BBB. So why does liver disease have an impact on brain function?
How the liver communicates with the brain during inflammation
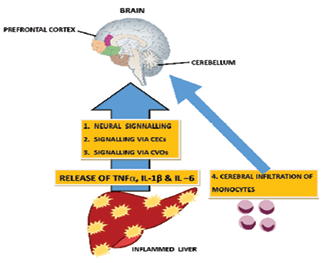
There are four potential communication pathways between the liver and brain. The pathways are the neural pathway, signalling via cerebral endothelial cells (CECs), signalling via circumventricular organs (CVOs) and cerebral infiltration of monocyte.
The neural pathway
The vagal afferent nerve innervates the liver. Cytokines such as TNFa (tumour necrosis factor-a), Interleukin-1β and interleukin-6 (IL-6) act directly to activate the vagal afferent nerve by binding to its cytokine receptors. The macrophage scattered between the vagal fibres within the nerve also responds to cytokines. Then the vagal afferent project to the dorsal vagal complex, which consists of nucleus tractus solitarius (NTS), area prostema and dorsal motor nucleus. Then later, it projects to the cerebral region of the brain, paraventricular nucleus (PVN) and the hypothalamus.
Signalling via CECs
The circulating cytokines (TNFa, IL-1β and IL-6) interact with receptors on CECs to mediate the activation of cells within the brain parenchyma to mediate sickness behaviour with liver inflammation.
Signalling via Circumventricular organs (CVOs)
The CVOs are the brain region that lacks an intact BBB. CVOs area consist of area postrema, medium eminence and organum vascualris of lamina terminalis. The capillaries in his region are fenestrated allowing molecules within the circulation to access the CVOs parenchyma. During inflammation, the cytokines (TNFa, IL-1β and IL-6) can access the brains via CVOs and choroid plexus, where these regions lack intact BBB).
Cerebral infiltration of monocyte
Monocyte transmigration into the brain in response to initial activation of resident cerebral microglial to produce monocyte chemoattractant protein 1 (MCP1). MCP1 is a pro-inflammatory chemokine is able to disrupt the integrity of BBB and further deteriorating the disease.
All these pathways mentioned above result in changes in central neural activity thereby altering the behaviour and leading to cognitive impairment, fatigue, and depression.
Cognitive impairment and depression
Prolonged high fat and high sugar diet and inactive lifestyle lead to progressive hepatic steatosis stimulating inflammation and proinflammatory cytokines activation. The presence of insulin resistance initiates the breaking down of lipid and lipid disequilibrium cascade leading to the production and accumulation of ceramides. These ceramides are cytotoxic and further promote insulin resistance, and their lipid solubility property enables them to be transferred across BBB, leading to brain insulin resistance (IR).
Brain IR causes neuronal loss and impaired neurotransmitter function for plasticity, learning and memory. It also impairs the oligodendrocyte’s survival and function, further enchancing neuroinflammation, oxidative stress and neurodegeneration.
In the early stage of chronic liver disease, the cerebellum is the first affected. Following the progression of brain injury, the hippocampus and prefrontal cortex (PFC), will be affected.
These areas are responsible for cognition, memory, learning and mood regulation. Some studies also reported that changes in neural transmission in basal ganglia impact motivational and reward behaviour, leading to central fatigue (tiredness).
So, what can we do to prevent this?
Although there are no medications to reverse fat accumulation in the liver, preventive action must be taken. Eat more fibre-rich foods such as fruits and vegetables, limiting each meal’s portion size or reducing 500 calories per day.
Limit the intake of saturated fats found in meat, poultry skin, shortening, milk and dairy products except for fat-free versions. All these saturated fat food products can be replaced with monounsaturated ones (olive, canola, avocado, peanut butter, and most nuts) and polyunsaturated ones (sunflower, corn, soybean and cottonseed oil). Monounsaturated and polyunsaturated food products are good for health.
Changing lifestyle is also one of the preventive measures that need to be taken into account. Exercising for 15 minutes three times per week would help maintain a healthy weight. Sleeping early is good for our liver health. The liver typically works to detox and cleanse the blood from 1am to 3am.
Therefore, keeping awake at that time will slow down the process. Thus, sleeping late at night can lead to poor metabolism, development of cancer, diabetes, and hypertension.
In conclusion, maintaining a healthy liver leads to maintaining good brain function. – The Health
Assoc Prof Dr Roslina Abdul Rahim lectures at the Kulliyyah of Medicine, International Islamic University Malaysia








