The Ministry of Health considers research and development an essential part of the healthcare system
BY KHIRTINI K KUMARAN
Since the pandemic broke out early last year, a significant portion of the world’s research output was diverted to understand the Covid-19 virus and its severity. Today, although we have developed vaccines to combat the virus, the battle is far from over, with mutant strains emerging.

Malaysia is one of the countries that have been part of the research and clinical trials. Dr Hishamshah Mohd Ibrahim, Deputy Director-General (Research & Technical Support) of the Ministry of Health (MoH), spoke with The Health on the research and development conducted and helmed by MoH, especially on the Covid-19 virus and its vaccines. He also talked about issues such as health management, the rising number of positive cases, vaccine rollout and the proposed National Vaccine Centre.
What is the latest research carried out by the MoH on the Covid-19 virus and its vaccines?
We’ve researched the virus and the vaccines in the National Institute of Health (NIH). There are about 88 ongoing research, 44 research grants and over 44 publications.
For example, at the Institute for Medical Research (IMR), we have been doing genome sequencing to identify new variants of the SARS-CoV-2 virus in Malaysia since last year. There are also collaborations with our other colleagues in the universities and Malaysia Genome Institute (MGI).
We have done about 336 whole-genome sequencings of the virus and identified several mutations, including the South African mutation, UK mutation, and the Indian double mutation. In IMR, they are also preclinical studies to develop two types of Covid-19 vaccines – mRNA vaccines and inactivated vaccines.
The studies that come under the ambit
of the Institute for Clinical Research (ICR) are:
• The vigilance study on post-Covid-19 vaccination immunogenicity surveillance among healthcare workers, which was rolled out in March of this year, is for two years.
• The Safe COVAC study is a case-based clinical safety study. It aims to assess whether there is an increased risk of specific adverse events following vaccination with Covid-19 vaccines.
Other studies include:
• A longitudinal study on neutralising antibodies among recovered Covid-19 patients 12 months after infection;
• Assessing saliva sampling of Covid-19 rapid tests antigens. Evaluating several test kits that use saliva sampling;
• Post-vaccination Covid-19 immunity and disease surveillance in Malaysia called the IMSURE study;
• Antibody response about hospitalised Covid-19 patients according to the severity and;
• Immune response after the first dose of Pfizer and Sinovac vaccines among recovered Covid-19 patients.
How do you obtain participants, and what are the challenges?
It is all voluntary as we advertise these studies to those willing to participate. They have to satisfy specific criteria. We have got the inclusion criteria and exclusion criteria for any proper research.
For example, we recently completed a phase three trial with 3,000 local participants for the inactivated vaccine developed by the Institute of Medical Biology of the Chinese Academy of Medical Sciences (IMBCAMS).
The participants are all volunteers who are healthy and fit the criteria. And we explain the study and have them sign the consent. Their identity and information are protected. They will be anonymised, whether they will be given either the vaccine candidate or placebo. The study is conducted in a blinded fashion. So, they’re going to be randomised, and that’s why we call it a randomised control trial.
How significant is our local clinical and behavioural research capabilities, and how does it contribute to health management in the country?
Under the National Institutes of Health (NIH), we have six research institutes: Institute for Medical Research (IMR), Institute for Clinical Research (ICR), Institute for Public Health (IPH), Institute for Health Systems Research (IHSR), Institute for Health Management (IHM) and Institute for Behavioural Research (IHBR).
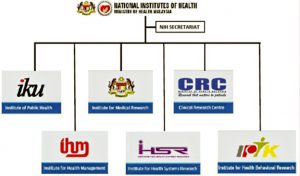
With regards to health management, behavioural research is critical, and this is done by the IHBR.
To date, they have done behavioural research such as why people smoke, usage of the internet for health information seeking, etc. There was behavioural research done on Covid-19 as well, such as on the SOP compliance and vaccine acceptance.
Findings from this periodical research, over time, give us a sense of whether the behaviour of our population has changed or not. And as we move forward, we can identify the kind of intervention that we need to do to make it more impactful and long-lasting.
We also have the Health Education Division (HED) under the MoH. So, there are usually collaborations between IHBR and HED. Apart from that, we also collaborate with the local universities and other researchers outside of MoH.
We have research collaborations with the World Health Organisation (WHO) because they have research capacity and funding. And we interact with them on a specific aspect of the research, which we want to highlight and study.
How important is research and development (R&D) to the MoH in managing healthcare issues in the country?
Medicine is a rapidly evolving field. Information and knowledge learned in medical school tend to become obsolete as the years go by. Thus, we cannot separate R&D from medicine as it is part and parcel of the healthcare system.
And in MoH, we value R&D because that is how we make sure that our people receive the best care for the whole range of service that we provide. Now, along with the entire country, we are looking into R&D innovation and commercialisation in healthcare.
When Covid-19 first appeared, IMR quickly developed sets of primers and probes even before any manufacturer came up with diagnostic tools.
Recently, we also created another research unit. The engineering unit developed the 3D printed ventilators and then the splitter for the ventilators to be used by two people.
Other research areas include precision medicine, health system research and utilising digital health, Artificial Intelligence (AI) and big data.
What is your take on the current situation where there is a daily rise of Covid-19 cases?
About two weeks after implementing MCO 2.0, we could see a decline in cases. We then started to ease restrictions by opening up schools, allowing mobility and travelling across States. The onus is on members of the public.
Although they can move about, they should strictly conform to the new norms and practice social distancing practices. But unfortunately, this is not happening, and social behaviour is challenging to control. Now, we can see the numbers rising again.
If the R Number (R0) does not go below 1 (one), there will be a transmission. There are concerns about whether our healthcare system can cope.
How effective are the vaccines against mutated variants of the virus?
According to the UK data, the AstraZeneca vaccine, whereby over 20 million doses have been inoculated, is still effective against the B117 variant. A study in Brazil shows that Sinovac is still efficacious against the P1 variant, which is quite virulent.
So, looking at the data from other countries so far, the current vaccines are still effective against the new variants. However, the efficacy may not be as high. It means the vaccine may not prevent infection, but it certainly will prevent severe disease and death.
However, there are concerns that the virus may further evolve to escape mutation, whereby it could evade immunity conferred by prior infections or vaccines.
When do you expect the vaccination rollout to end?
The initial aim was to complete the target by February next year. But suppose the manufacturers can ramp up the production and give us the supplies. In that case, we could probably do it by the third quarter or latest by the fourth quarter this year.
We hope we can reach the target by the third quarter and we have made sure to procure more than 100 per cent of the population. This ensures that we have a buffer because we never know when we will be required to give more.
What is your view on the proposal to establish a National Vaccine Centre (NVC), presented by MOSTI under the 12th Malaysia Plan?
I think it is long overdue. We need to be self-sustaining and not rely on China, Germany, the USA or Europe to get vaccine supplies.
However, it will require a lot of capital expenditure, and it will take some time. A few areas need to be developed: the preclinical studies platform; facilities for animal testing; facilities for clinical trial phase 1, phase 2, phase 3 and post-marketing phase 4; the regulatory aspect; and the vaccine manufacturing facilities.
It should be a collaborative effort. Though the NVC may be the main centre, the components may be spread out amongst all the other agencies such as MoH, IMR and National Pharmaceutical Regulatory Agency (NPRA). — The Health