More research is needed to determine the optimum option for e-cigarette quitting


Electronic nicotine delivery systems (ENDS), also known as electronic cigarettes (e-cigs), are increasingly used in Malaysia among adults and adolescents.
The 2019 National Health and Morbidity Survey (NHMS) reported 1.1 million e-cig smokers or adult vapers, compared to 600,000 or 3.2 per cent vapers found in 2016 from the National Electronic-Cigarette Survey (NECS). Alarmingly, the 2022 NHMS found an increase in vaping among Malaysian adolescents between 13-17 years old, from 9.8 per cent in 2017 to 14.9 per cent in 2022 with 23.3 per cent males and 6.2 per cent among females.
Vapers dependent on nicotine, the main addictive chemical in ENDS, often exhibit craving and withdrawal symptoms upon temporary or abrupt cessation of vaping, similar to those experienced by smokers with tobacco use disorder (TUD). According to the 5th Edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-5), TUD is diagnosed when a person uses tobacco for more than 12 months, with a minimum of two of the 11 following features:
1. More amounts of tobacco over a longer time frame than planned
2. Inability to quit or lessen the amount of tobacco use despite efforts to do so
3. An excessive amount of time spent on attaining or using tobacco
4. Craving or a strong desire or urge to use tobacco
5. Relinquishing responsibilities because of tobacco use
6. Persistent use of tobacco despite its negative impact both socially and in relationships
7. Abandoning career, social, and other activities to use tobacco
8. Use of tobacco in harmful situations/settings
9. Persistent use of tobacco, even in the face of physical or emotional difficulties related to tobacco use
10. Tolerance, as defined by either the need for markedly increased amounts of tobacco to achieve the desired effect or a markedly diminished effect with continued use of the same amount of tobacco
11. Withdrawal, as manifested by either the characteristic withdrawal syndrome or the use of tobacco to relieve or avoid withdrawal symptoms
Tobacco withdrawal symptoms which appear upon cessation include craving, irritability, mood changes, trouble sleeping and increased appetite. Most of these symptoms peak in the first few days and gradually dissipate around four to eight weeks. The majority of vapers, similar to conventional cigarette smokers, cannot stop due to the intensity of urges and withdrawal symptoms which are correlated to the severity of nicotine dependence.
Based on the Malaysian Clinical Practice Guidelines Treatment of TUD 2016, approved first-line pharmacological intervention for conventional cigarettes includes nicotine replacement therapy (NRT) products (gums, patches, etc) and varenicline. These medications help to reduce craving and withdrawal symptoms to aid successful cessation.
All forms of NRT increase the probability of successful cigarette quitting attempts. Generally, monotherapy NRT increase the chances of successful abstinence by 50 per cent to 70 per cent, and it is similar between products.
Monotherapy varenicline is shown to yield about 50 per cent higher cessation rate than monotherapy NRT. Still, evidence shows that the combination of two NRT products, e.g., gum plus patch, yield similar efficacy as monotherapy varenicline.
Pharmacological and behavioural interventions
For best results, pharmacotherapy for TUD is combined with behavioural intervention. Intensive behavioural support for smokers using cessation medication improves the probability of long-term abstinence by approximately 10 per cent to 25 per cent.
E-cigs mimic the rapid delivery of nicotine by combustible cigarettes, yielding high nicotine levels in the plasma. Vapers are also reported to titrate or compensate their nicotine needs by adjusting the nicotine concentration of the liquid and the ‘topography’ of vaping, i.e., by inhaling deeper, taking more puffs, and breath-holding.
Like cigarette smokers, rates of metabolising nicotine and numbers of nicotinic acetylcholine receptors (nAchRs) in the brain also affect the topography of vaping. More nicotine is consumed by fast metabolisers than slow metabolisers, as well as in those with higher number of upregulated nAchRs due to nicotine intake.
Related to these, nicotine withdrawals from vaping have been described both in adults as well as adolescent vapers. Hence, it seems logical that both pharmacological and behavioural interventions approved for TUD should also be beneficial for e-cig cessation.
However, unlike cigarette smoking cessation where protocols exist for assessment of dependence and severity of withdrawals, drug selection, dosing and duration, as well as for special populations including adolescents, it is not so apparent for e-cig cessation.
There have been few questionnaires developed to assess dependence among adult vapers, including Fould’s Penn State (Electronic) Cigarette Dependence Index and Fagerstrom Test for Nicotine Dependence (FTND) modified for e-cigs, where time to first cigarette (TTFC) is replaced with time to first vape and number of cigarette/day is substituted by number of puff/session and number of session/day.
However, it has been reported that most vapers are unsure of the number of puffs and sessions vaped per day. Additionally, the nicotine concentration used vary greatly among vapers, with rampant mislabelling.
For adolescents, the Hooked on Nicotine Checklist (HONC) adolescent (cigarette) smoking dependence questionnaire has been edited for adolescent vapers.
Assistance for vaping cessation
The use of NRT for e-cig smoking cessation seems intuitive, and adaptation for vaped nicotine withdrawals seems plausible. However, it is still off-label for such use.
As with smoking cessation, NRT underdosing occurs frequently. Therefore, mono or combination therapy of NRT products can be considered in vapers according to their level of dependence and adjusted to the severity of withdrawal symptoms.
Vapers with high nicotine dependence can start with a 21mg patch per day, while lesser dependence was recommended with a 14mg patch, subsequently tapered down until the participants stopped vaping.
Some studies utilised 2mg or 4mg of nicotine lozenge and gum with no tapering, or nicotine nasal spray. Side effects reported by study participants include itching and redness at the site of nicotine patch application, and heartburn in those who used nicotine gum. All studies that employed NRT reported participants successfully quit vaping after three and six months.
Varenicline has been confirmed to be effective during the three months follow-up by successfully preventing participants from smoking e-cigarettes totally, resulting in improved health. The subjects also reported having minor side effects, including vivid dreams and weight gain.
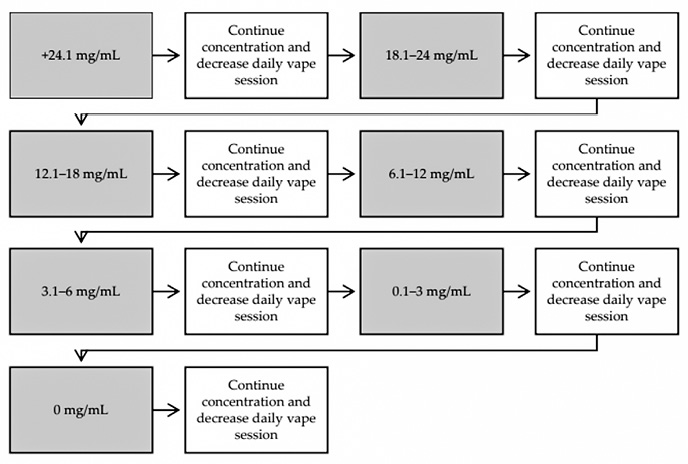
Tapering of nicotine concentration in e-cig as a method of quitting has been reported. A pilot study found that the efficacy of the vape-taper method is comparable to NRT (Sahr, Kelsh, & Blower, 2019).
A text message vaping cessation intervention was found to be effective in promoting dual abstinence from e-cigarettes and combustible cigarettes among young adults. Such intervention provided participants with encouragement and support, skill training, self-efficacy building exercises, coping methods, and information about the risks of vaping and the benefits of stopping for nine weeks after they selected their quit date.
The advantage of this intervention over control was equivalent for exclusive e-cigarette users and dual users. Rates of dual abstinence were higher among exclusive vapers than dual users, suggesting the importance for further research for poly-tobacco users.
Behavioural interventions for quitting combustible cigarettes such as practical counselling (problem solving or skills training) and intra-treatment social support is an integral part of e-cigarette cessation intervention while waiting for approved e-cig medications.
Conclusion
Evidence on vaping assessment tool and cessation interventions are currently limited. Nicotine replacement treatment products and varenicline have been shown to increase vaping cessation with minor side effects. No medication is currently approved for e-cigarette cessation.
Vape-taper is also useful while interactive text message demonstrated significant success in e-cigarette cessation. Counselling successfully assisted in vaping discontinuation by pairing it with other interventions.
More research is needed to determine the optimum option for e-cigarette quitting, including in special populations. – The Health
Dr Mohamad Haniki Nik Mohamed is Professor, Pharmacy Practice Department, Kulliyyah of Pharmacy, IIUM and the Head, Smoking Cessation Task Force, Academy of Professors Malaysia. He is also the Past President of the Malaysian Association of Adolescent Health.








